한국 성인의 철 결핍성 빈혈과 근시와의 관련성
Association between Anemia and Myopia in Korean Adults
Article information
Abstract
목적
철 결핍성 빈혈(iron deficiency anemia, IDA)과 안질환 사이의 관계를 증명한 기존 연구들이 있지만, IDA와 근시와의 관련성에 대한 연구는 아직 보고되지 않았다. 본 연구는 IDA와 근시와의 관계를 조사하기 위해 실시되었다.
방법
국민건강영양조사(2010-2012)의 자료를 이용하여 20-54세 성인 8,672명을 대상으로 하였다. IDA는 혈색소(hemoglobin, Hb) 농도가 남성 13 g/dL 미만, 여성 12 g/dL 미만, 임산부 11 g/dL 미만으로 정의하였다. 추가로 Hb 농도에 따라 IDA를 경증과 중등도 및 중증으로 세분화하여 분석하였다. 근시는 구면렌즈대응치가 -0.5 diopters 이하인 경우로 정의하였다. IDA와 근시와의 관련성을 알아보기 위해 공변량을 보정 후 복합표본 다중 로지스틱 회귀분석을 시행하였다.
결과
근시 유병률은 남성이 43.5%, 여성이 56.5%로 여성이 남성보다 유의하게 높았다. IDA가 있는 여성은 IDA가 없는 여성에 비해 근시의 교차비가 1.28배 높았다(odds ratio, OR=1.28, 95% confidence interval, CI=1.02-1.60, p =0.036). IDA의 중증도에 따라 근시와의 관련성을 분석한 결과, IDA가 없는 여성에 비해 중등도 및 중증 IDA (Hb <11 g/dL)를 가진 여성은 근시의 교차비가 1.55배 높았다(OR=1.55, 95% CI=1.12-2.16, p =0.026). 남성의 경우 IDA와 근시와의 관계는 통계적으로 유의하지 않았다.
결론
IDA가 있는 한국 성인에서 중등도 및 중증 IDA를 가진 여성은 근시 유병률과 유의한 연관성이 있음을 확인하였다.
Trans Abstract
Objectives
Anemia is a significant public health issue worldwide and has been associated with ocular changes. This study aimed to investigate the relationship between anemia and myopia in an adult population in South Korea.
Methods
We analyzed the data of 8,672 participants aged 20 to 54 years in the fifth Korea National Health and Nutrition Examination Survey (KNHANES). Anemia was defined as hemoglobin levels <13 g/dL for men, <12 g/dL for women, and <11 g/dL for pregnant women. Anemia was subdivided into mild anemia (11-12.9 g/dL for men, 11-11.9 g/dL for women, and 10-10.9 g/dL for pregnant women) and moderate to severe anemia (<11 g/dL for men and women and <10 g/dL for pregnant women) categories. Myopia was defined as spherical equivalent value ≤-0.5 diopters. We performed multivariable logistic regression analysis to examine the relationship between anemia and myopia.
Results
The prevalence of myopia was 43.5% in men and 56.5% in women. The prevalence of anemia was 0.9% in men and 14.5% in women. After adjusting for confounders, the risk of myopia was 1.28 times higher in women with anemia than in those without anemia (odds ratio, OR=1.28, 95% confidence interval, CI=1.02-1.60, p =0.036). The risk of myopia was 1.55 times higher in women with moderate to severe anemia than in those without anemia (OR=1.55, 95% CI=1.12-2.16, p =0.026). Anemia was not significantly associated with myopia in men.
Conclusions
Women with anemia were at increased risk of myopia compared to those without anemia. The severity of anemia was also associated the risk of myopia.
INTRODUCTION
Anemia has been considered a major public health issue that affects 1.62 billion people (24.8%) worldwide [1]. Anemia can cause an economic burden for individuals and their families [2]. In Southeast Asia, the risk of anemia is high, with about 40% of non-pregnant women and about 30% of pregnant women having it [1]. According to a Global Burden of Diseases, Injuries, and Risk Factors Study 2010, anemia was consistently more prevalent women than in men until adulthood [3].
Myopia is attributed to excessive refractive power or increased axial length of the eye [4]. The prevalence of myopia is rapidly increasing throughout the world, and it occurs most in countries of East Asia [5,6]. For example, the prevalence of myopia was 48.1% in adult Koreans [7], 41.8% in elderly Japanese [8], and 38.9% in adult Singaporeans [9]. The number of people with myopia is expected to reach approximately 5 billion by 2050, accounting for about half of the world's population [5].
Previous studies have reported that anemia is related to ocular changes. For example, retinal vascular tortuosity was directly associated with the severity of anemia [10]. Anemia with hemoglobin (Hb) concentrations less than 8 g/dL patients increased the prevalence of retinopathy [11]. As a type of anemia, iron deficiency anemia (IDA) increases concentrations of hemoglobin A1c (HbA1c) levels [12-15]. HbA1c concentrations are associated with refractive error, especially myopia [16,17]. Moreover, IDA patients had a significantly decreased choroidal thickness [18] which is associated with myopia [19].
Several studies have demonstrated the relationship between anemia and eye-related problems [10,11,18]; however, there was no study investigating the relationship between anemia and myopia. In the basis of adverse effects of anemia on ocular changes, we hypothesized that anemia status was associated with the risk of myopia; the risk of myopia was different according to the severity of anemia. We tested two hypotheses in Korean adults using nationwide survey data.
METHODS
Study population
The fifth Korea National Health and Nutrition Examination Survey (KNHANES) included 25,534 participants (11,616 men and 13,918 women). The prevalence of myopia increases in early adulthood and then decreases over the age of 50 years [20]. Thus, this study set the age of participants from 20 to 54 years, referring to previous studies [21,22]. Since only the fifth KNHANES met the age group of 20-54 years and measured myopia, we used the data from the fifth KNHANES in this study. Among 25,534 participants, 20 to 54 years, who underwent ophthalmologic examinations and having measured serum Hb were selected. Participants who had previous ocular surgery, such as cataracts, glaucoma, or refractive surgery, and missing serum Hb data were excluded. Ultimately, 8,672 participants (3,742 men and 4,930 women) met the inclusion criteria.
Material
Ophthalmic examination and anemia
Refraction was measured by using an autorefractor and Keratometer (KR-8800, Topcon, Tokyo, Japan) of both eyes by ophthalmologists. The refractive error was converted into a spherical equivalent (SEQ), calculated by the sum of the spherical value and half of the cylinder value. Myopia was defined by the SEQ of -0.5 diopters (D) or less based on the left eye [23].
Blood samples were collected in the morning after overnight fast. Hb concentration measurements were made using a sodium lauryl sulfate method with an automated hematology analyzer XE-2100D (Sysmex, Kobe, Japan). Anemia was defined as Hb <13 g/dL for men, <12 g/dL for women, and <11 g/dL for pregnant women following the guidelines of the World Health Organization [24]. Additionally, anemia was subdivided into mild anemia (11-12.9 g/dL for men, 11-11.9 g/dL for women, and 10-10.9 g/dL for pregnant women) and moderate to severe anemia (<11 g/dL for men and women and <10 g/dL pregnant women) categories.
Covariates
Demographic, socioeconomic, lifestyle, and eye-related information was collected by trained interviewers. The following variable attributes were used in this study: age (continuous, year), body mass index (BMI; continuous, kg/m2), residential area (urban or rural), income (low, low-moderate, moderate-high, or high), education (≤middle school, high school, or ≥university), occupation (white-collar worker, blue-collar worker or unemployed with students and full-time housewives), current smoking status (current smoking at least 100 cigarettes in all life or not), alcohol consumption status (at least once a month in the past year or not), diabetes mellitus (fasting plasma glucose ≥126 mg/dL/taking hypoglycemic agents or not), stress (yes or no), moderate-intensity physical activity (≥20 minutes per session and ≥3 days/week or not), daily riboflavin intakes (continuous, mg), vitamin D level (continuous, μg), sun exposure (<2 hours, 2-5 hours, or ≥5 hours), and family history of eye disease (yes or no). The covariates were classified into socio-demographic and health-related variables (BMI, residential area, household income, education level, occupation, current smoker, alcohol consumption, diabetes mellitus, stress, moderate-intensity physical activity, daily riboflavin intakes, and vitamin D level), and eye health-related variables (sun exposure time and family history of eye disease).
Statistical analysis
The participants were classified into myopic and non-myopic by sex. A t-test was used for continuous variables for comparison between two groups; a Chi-square test was used for categorical variables. After adjusting for confounding variables, multivariable logistic regression analysis with complex sample design was performed to evaluate the relationship between anemia and myopia. All statistical analysis were performed by Proc Surveyfreq, Proc Surveymeans, Proc Surveyreg, and Proc Survey-logistic with SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value <0.05 was considered to be statistically significant.
Ethical approval
We performed this study using the fifth KNHANES 2010-2012 data. The KNHANES has been surveyed nationwide since 1998 to obtain representative data on the health and nutritional status of the general South Korean population. A representative sample of the Korean population is drawn from a stratified multistage cluster sampling. The survey was conducted by the Korea Disease Control and Prevention Agency (KDCA), and trained investigators conducted health interviews with a questionnaire, nutritional examinations, and health examinations. The KNHANES was approved by the Institutional Review Board (IRB) of the KDCA, and all participants signed an informed consent form to provide their data for research. The study was carried out following the Declaration of Helsinki. Our study protocol was exempted from the ethical review by the IRB of the University of Seoul (IRB No.: UOS 2021-04-003).
RESULTS
The prevalence of myopia was 43.5% in men and 56.5% in women (Table 1). The mean age of the participants with myopia was 36.0 years for men and 36.4 years for women. Participants with myopia, in both men and women, were significantly younger, lived in the city, more educated, had a higher proportion of white-collar workers and unemployed, a lower proportion of blue-collar workers, and less exposed to the sun than those without myopia. In men, participants without myopia (1.5%) were more anemic than those with myopia (0.7%, p =0.045). In women, participants with myopia (14.9%) were more anemic than those without myopia (11.7%, p =0.008).
The prevalence of myopia was 0.7% (n=19) in men with anemia and 14.9% (n=484) in women with anemia, which was higher among women than men (Table 2). Participants with anemia had a significantly lower prevalence of myopia compared to those without anemia for both men and women.
Myopia was more likely in women with anemia than those without anemia (OR=1.32, 95% CI=1.07-1.62, p =0.009) (Table 3). These findings remained after adjusting for covariates; women with anemia had 1.28 times higher risk of myopia than non-anemia women (OR=1.28, 95% CI=1.02-1.60, p =0.036). However, there was no significant association between anemia and myopia in men.
We also analyzed the risk of myopia according to the severity of anemia. Among women, the risk of myopia was 1.55 times higher for those with moderate to severe anemia compared to those without anemia (OR= 1.55, 95% CI=1.12-2.16, p =0.026) (Figure 1). Meanwhile, women with mild anemia did not have an increased risk of myopia compared to women without anemia. In men, none of the associations between anemia and myopia was identified.
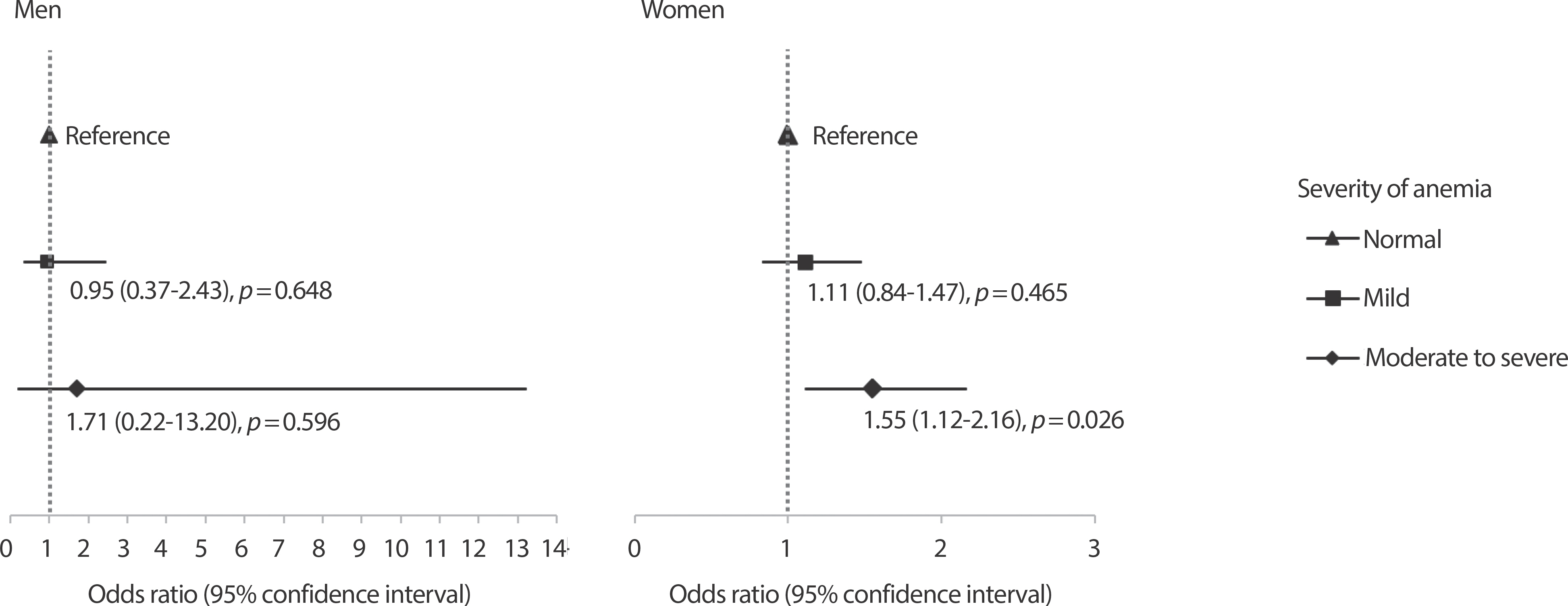
Adjusted odds ratio (95% confidence interval) for myopia according to severity of anemia. The model adjusted for age, body mass index (kg/m2), residential area, household income, education, occupation, current smoker, alcohol consumption, diabetes mellitus, stress, moderate-intensity physical activity, daily riboflavin intakes, vitamin D level, sun exposure time, and family history of eye disease.
We performed subgroup analysis only for women, because of very low prevalence of anemia in men (0.9%, n=35). With full-adjustments, the risk of myopia was significantly higher in women aged 40-54 years with anemia than in those without anemia (OR=1.47, 95% CI=1.11-1.94, p = 0.007), but no significant difference was found in the relationships between anemia and myopia in women aged 20-39 years. The relationship between anemia and myopia was significant only for women who graduated high school (OR=1.63, 95% CI=1.18-2.24, p =0.003) or who did not perform moderate-intensity physical activity (OR=1.32, 95% CI= 1.02-1.69, p =0.032).
DISCUSSION
Our study showed that women with anemia had an increased risk of myopia. The risk of myopia was higher in women with moderate to severe anemia than in those without anemia. Through further analysis, we found that the relationship between anemia and myopia was significant in women aged 40-54 years, high school graduates, and physically inactive. However, no significant association was found for men in any of the analysis.
Few studies have directly observed that anemia affects visual acuity. Kacer et al. [25] reported that 2 patients of retinal vessel occlusion in mild IDA were associated with ophthalmologic complications. The patients had Hb concentrations of 9.4 g/dL and 7.3 g/dL, respectively. Despite systemic steroids treatment, the visual acuity was gradually worsened. Consequently, the authors noted that patients with chronic IDA should be aware of possible ophthalmic manifestations [25].
Although the mechanism of anemia that affects myopia remains un-clear, several mechanisms can be suggested. First, low Hb concentrations may reduce choroidal thickness and cause myopia. Hb concentrations and choroidal thickness were significantly correlated in women with IDA, and those patients had significantly reduced choroidal thickness [18,26]. Highly myopic patients tended to have thinner choroidal thickness compared to controls [27]. The choroid receives the greatest ocular blood flow and supplies oxygen to the outer layers of the retina [28]. Simsek et al. [26] suggested that IDA leads to decrease iron and ferritin levels and hypoxia, which can interrupt the choroidal structure that blood flow to the eye and such interruption may affect other forms of ocular disorders.
Second, increased HbA1c levels in anemic people may contribute to the development of myopia. In iron deficiency status, the HbA1c level is elevated due to the red cell production rate decreasing [29]. A previous study showed that IDA and iron deficient state women had significantly higher HbA1c levels than normal iron state women [30]. In late pregnancy, HbA1c levels were elevated due to iron deficiency [12]. Elevated HbA1c levels may increase the risk of myopia [16]. Another study showed that patients with HbA1c levels ≥8.8 had a 60% increased risk for myopic shift compared to HbA1c levels <8.8 [31].
In this study, we found that anemia was associated with myopia only in women. Several mechanisms may be suggested to explain the sex difference. First, the small number of patients with anemia in men (n=35) may have resulted in non-significant association between anemia and myopia in men. Second, thicker choroids in men than in women [32,33] may have attenuated the effects of anemia in causing myopia in men. Because the men's choroidal thickness is relatively thicker, even if they have anemia, their choroidal thickness may have been less affected. Several studies have reported sex differences in choroidal thickness and it is explained by the lower basal sympathetic tone in men and the sex hormonal status that may influence choroidal blood flow [34,35]. Third, the potential mechanism that iron deficiency may have induced myopia through HbA1c may work only in women. A previous study showed that iron deficiency increased the odds of having an HbA1c ≥5.5% in women, but there was no significant relationship between iron deficiency and HbA1c [36].
We speculated that riboflavin might cause a confounding effect between anemia and myopia. A previous study showed that the women with riboflavin biomarker (erythrocyte glutathione reductase activity coefficient) deficiency had 2-fold greater odds of anemia than those without deficiency [37]. Insufficient riboflavin status is associated with iron handling and plays a role in the etiology of anemia when iron intakes are inadequate [38]. Riboflavin is also assumed to be associated with myopia. Oral riboflavin and whole-body ultraviolet A irradiation decreased myopia progression in a guinea pig experiment [39]. Accordingly, we additionally adjusted for riboflavin in statistical models, but the relationship between anemia and myopia was still significant. This means that even after excluding the effects of riboflavin, the relationship between anemia and myopia remained.
Our study has several limitations. First, it is difficult to draw causal inferences between anemia and myopia, because KNHANES is a cross-sectional design. Therefore, the possibility of an inverse association cannot be ruled out. To demonstrate causality, a randomized controlled trial study will be needed on a large number of populations. Second, the study population included people aged between 20 and 54 years; therefore, the research findings could not be generalized to children, adolescents, and people aged 55 years and older. Third, the fifth KNHANES did not survey some of potential confounders (e.g., parental history of myopia and close range work), we could not adjust them in the statistical model. Fourth, KNHANES measured refractive error without using cycloplegic agents, so the prevalence of myopia could be overestimated or underestimated. Fifth, Hb concentrations were measured at a single time point, which may affect the precision and accuracy of diagnosing anemia. Despite these limitations, this study is the first study to explore the relationship between anemia and myopia. Moreover, because we tested our hypothesis using a nationwide representative population of Koreans, our findings can be generalized to Korean adults aged between 20 and 54 years.
In conclusion, women with anemia were at increased risk of myopia compared to those without anemia. The risk of myopia was higher in women with moderate to severe anemia than in those without anemia. However, anemia was not significantly associated with myopia in men. Further epidemiology and experimental studies are needed to confirm the relationship between anemia and myopia and to elucidate the underlying mechanisms in this relationship.



